What is PDMP (Prescription Drug Monitoring Program)?
A Prescription Drug Monitoring Program (PDMP) is a state-run electronic database that tracks the prescribing and dispensing of controlled substances. PDMPs are designed to help healthcare providers, pharmacists, and regulators identify potential misuse, prevent “doctor shopping,” and promote safer prescribing practices.
Most U.S. states operate their own PDMP, each with specific requirements for when and how prescribers must check the system. PDMPs are a critical tool in efforts to address the opioid crisis, improve patient safety, and reduce prescription drug abuse, while still ensuring that patients with legitimate medical needs receive appropriate treatment.
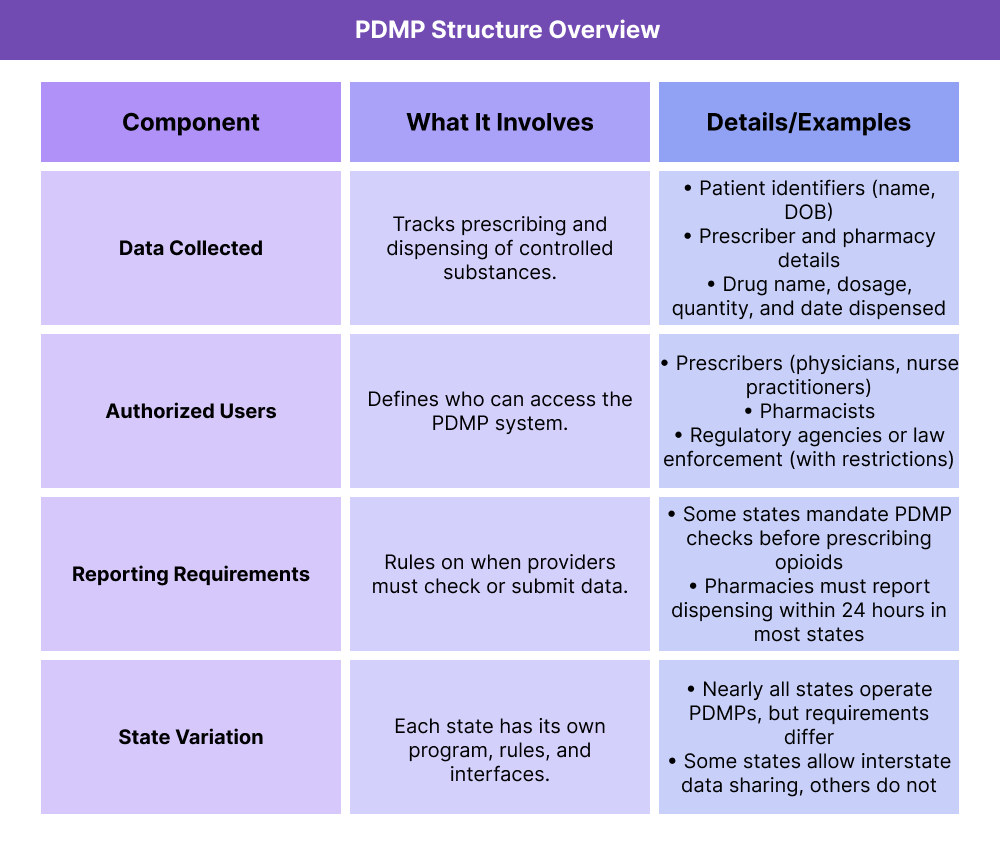
Key Components of a Prescription Drug Monitoring Program
While each state designs and manages its own Prescription Drug Monitoring Program (PDMP), most share the same core elements. These include the type of data collected, who can access the system, and the reporting rules that govern its use.
What Data Do PDMPs Collect?
PDMPs compile real-time prescription data on controlled substances, including drug type, dosage, prescriber, pharmacy, and patient identifiers. This helps providers spot overlapping prescriptions or risky medication patterns.
Who Has Access to PDMPs?
Authorized users usually include prescribers, pharmacists, and (with restrictions) regulators or law enforcement. Providers must follow strict privacy and compliance rules when accessing patient records.
What Are PDMP Reporting Requirements?
Many states require prescribers to check the PDMP before writing new prescriptions for opioids or other controlled medications. Pharmacies are typically required to report dispensing data within 24 hours, ensuring the system stays up to date.
Why Do PDMP Rules Differ by State?
Each state operates its own PDMP, meaning requirements vary widely. Some states allow interstate data sharing to track prescriptions across borders, while others keep data limited to in-state use.
How Prescription Drug Monitoring Programs Work in Practice
PDMPs operate as real-time monitoring systems that track the prescribing and dispensing of controlled substances. The process involves providers, pharmacists, and state-managed databases working together to ensure safe, compliant prescribing.
Step 1 — Prescription Is Written
A healthcare provider writes a prescription for a controlled substance. In many states, the provider must check the PDMP database before finalizing the prescription to ensure the patient is not receiving overlapping or risky medications.
Step 2 — Prescription Is Dispensed
When a pharmacy fills the prescription, it must report the transaction (drug, dosage, prescriber, patient, and date) to the state PDMP system. Most states require reporting within 24 hours.
Step 3 — Data Is Stored in PDMP Database
The PDMP system collects and organizes prescription data into a state-run database. Some states also participate in interstate data sharing to track patients who cross state lines.
Step 4 — Providers Access PDMP Data
Before prescribing or refilling controlled substances, providers can search the PDMP database to see the patient’s history. This helps identify potential issues like doctor shopping, overlapping prescriptions, or high opioid use.
Step 5 — Compliance and Oversight
Regulators and law enforcement (with restrictions) may use PDMP data to monitor prescribing patterns, detect fraud, or investigate misuse. This oversight adds another layer of accountability.
PDMPs and Their Impact on Billing and Reimbursement
While Prescription Drug Monitoring Programs (PDMPs) do not directly determine Medicare or Medicaid reimbursement rates, they play a critical role in compliance, prescribing safety, and regulatory oversight. Providers who fail to use PDMPs as required by law may face penalties, audits, or disciplinary action that can ultimately affect revenue and participation in federal programs.
Compliance with State and Federal Rules
- Many states require providers to check the PDMP before prescribing opioids or other controlled substances.
- Failure to comply can result in fines, sanctions, or loss of prescribing authority, which may indirectly affect reimbursement.
Integration with Promoting Interoperability (PI)
- Under the CMS Promoting Interoperability program, checking PDMPs can count toward certain E-Prescribing objectives.
- Proper PDMP use supports a provider’s PI score, which in turn impacts their overall MIPS composite score under the Quality Payment Program (QPP).
Risk Management and Liability
- Consistent PDMP use helps providers reduce the risk of overprescribing and protects against liability in cases of opioid misuse or diversion.
- Demonstrating compliance with PDMP requirements may help safeguard revenue by avoiding malpractice claims or payer audits.
Why PDMPs Matter in Billing and Reimbursement
Although PDMPs are not a billing mechanism, they are compliance-driven tools that directly influence how providers prescribe, report, and demonstrate responsible opioid management. Indirectly, they help providers maintain eligibility for value-based care programs and avoid financial penalties tied to non-compliance.
Frequently Asked Questions about PDMPs
1. What is a PDMP?
A Prescription Drug Monitoring Program (PDMP) is a state-run electronic database that tracks prescriptions for controlled substances. It is used to help prevent misuse, improve patient safety, and support safer prescribing practices.
2. Who can access PDMP data?
Authorized users typically include prescribers (such as physicians, nurse practitioners, and dentists), pharmacists, and in some cases regulators or law enforcement with restrictions. Access is controlled by each state’s program rules.
3. Do all states have PDMPs?
Nearly all U.S. states operate a PDMP, though program design, rules, and technology vary. Some states also share PDMP data across borders, while others limit access to in-state systems only.
4. How do PDMPs impact prescribing?
In many states, prescribers are legally required to check the PDMP before prescribing opioids or other high-risk medications. This helps identify possible doctor shopping, overlapping prescriptions, or unsafe medication combinations.
5. Are PDMPs required by law?
Yes, in most states. Requirements differ, but many mandate that prescribers check the PDMP before issuing certain prescriptions, and pharmacies must submit dispensing data within 24 hours.
6. How do PDMPs affect billing or reimbursement?
PDMPs do not directly change Medicare or Medicaid reimbursement. However, they support compliance with Promoting Interoperability (PI) requirements, which contribute to MIPS scoring under the Quality Payment Program (QPP). Failure to use PDMPs correctly may lead to regulatory penalties, liability risks, or downstream financial consequences.
