CPT 98976 Description, Billing Rules, & Use Cases
CPT 98976 is used to report the supply of a medical device that collects and transmits data to monitor a patient’s respiratory system as part of a Remote Therapeutic Monitoring (RTM) program.
The code applies to a 30-day service period and supports proactive care for patients managing chronic or recovering respiratory conditions.
What is CPT Code 98976?
CPT Code 98976 covers the provision of a medical device used for remote therapeutic monitoring of the respiratory system, including the device’s setup for scheduled recordings or programmed alerts. This code is billed once per 30-day period and requires a minimum of 16 days of monitoring to qualify.
The device supplied must be an FDA-defined medical device that either:
- Automatically records respiratory status data at scheduled intervals, or
- Triggers alerts when certain thresholds or conditions are met.
Once supplied and active, the device transmits data to the care team to track therapy adherence and patient response, enabling providers to intervene earlier and improve outcomes. This code applies only to the device supply component — any treatment or data management services are reported separately under codes like 98980 or 98981.
CPT 98976 Time Thresholds and Code Combinations
CPT 98976 is used to report the monthly device supply and monitoring activation for a Remote Therapeutic Monitoring (RTM) episode involving the respiratory system. It is typically billed once per 30-day period, provided specific technical and compliance conditions are met.
Important to Note:
CPT 98976 may be billed once per calendar month if all the following criteria are met:
- The device meets the FDA definition of a medical device
- It is capable of transmitting respiratory system data via scheduled recordings or alerts
- The device was supplied and activated by the billing provider or care team
- A minimum of 16 days of monitoring occurred within the 30-day period
- The service was ordered by a physician or qualified healthcare provider
- The billing documentation confirms device use and the start of a defined RTM episode
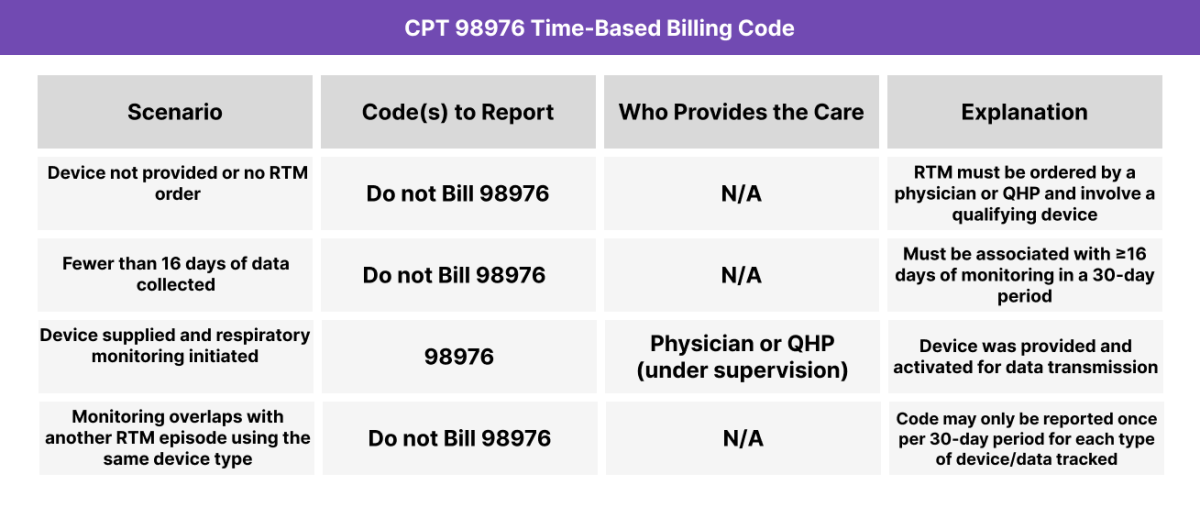
When to Use CPT 98976: Common Scenarios and Use Cases
CPT 98976 should be used when a provider or supervised clinical staff initiates a 30-day episode of respiratory monitoring by supplying a qualifying medical device. The code applies only if the device meets FDA standards, is configured for respiratory tracking, and collects at least 16 days of data within the billing period.
Here are examples of how CPT 98976 is used in practice:
CPT 98976 Billing Requirements and Eligibility
CPT 98976 is used to report the monthly provision of a device that monitors a patient’s respiratory status remotely. This includes the supply and configuration of a qualifying medical device that transmits scheduled recordings or alerts related to respiratory function, allowing providers to track adherence or therapeutic response.
This code may only be billed once per 30-day period and must meet the following requirements:
Patient Eligibility Criteria
- The patient must be actively managed for a condition that requires remote therapeutic monitoring of respiratory function, such as:
- Chronic obstructive pulmonary disease (COPD)
- Asthma or reactive airway disease
- Post-COVID respiratory complications
- Home ventilator weaning or monitoring programs
- The patient must understand and consent to device use, and must be engaged in a treatment plan that benefits from remote monitoring data to assess therapy progress or adherence.
Device and Setup Requirements
- To bill CPT 98976, the device must:
- Qualify as an FDA-defined medical device
- Be capable of transmitting respiratory-related data through scheduled recordings or programmed alerts
- Be provided to the patient for use in a home or outpatient setting
- The provider or supervised clinical staff must:
- Assign and activate the device
- Instruct the patient or caregiver on proper use
- Document all setup and transmission parameters
Timing and Episode Requirements
- CPT 98976 may be reported:
- This code cannot be billed if another code already covers the same monitoring activity for the same 30-day span.
Who Can Bill CPT 98976
CPT 98976 may be billed by:
- Physicians
- Nurse Practitioners (NPs)
- Physician Assistants (PAs)
- Other Qualified Healthcare Professionals (QHPs)
- Non-physician clinical staff operating under general supervision, when allowed by payer policy
Billing must be tied to a provider responsible for oversight of the RTM episode and the interpretation of transmitted data.
CPT 98976 Billing Documentation Checklist
CPT 98976 requires clear documentation that a qualifying device was supplied and that it is actively used to monitor respiratory function during a new or ongoing RTM episode. To support compliant billing, the patient’s record should include the following:
- A documented order from a physician or qualified healthcare provider:
- Must state the intent to begin remote therapeutic monitoring for a respiratory condition
- Should specify the device to be used and the clinical objective
- A description of the RTM device assigned to the patient:
- Confirmed to meet the FDA definition of a medical device
- Designed to capture and transmit respiratory system data (e.g., airflow, symptoms, breathing patterns)
- Uniquely assigned to the patient
- A statement confirming that the device was supplied and activated:
- Who provided the device (provider or supervised staff)
- When the supply and setup occurred
- That the device was enabled to record or transmit data
- Patient education (if applicable to the care model):
- Who delivered the training
- Whether it occurred in person or via telehealth
- What was reviewed (e.g., device use, troubleshooting, alert response)
- Identification of the 30-day monitoring period:
- Must reflect at least 16 days of expected data capture
- Distinguish this monitoring from any overlapping RTM services
- Confirmation that the patient:
- Was instructed on how and when to use the device
- Understood the transmission schedule (e.g., daily, alert-based)
- Consented to participate in the RTM process
- A follow-up tracking plan:
- Who on the care team will monitor incoming data
- When data will be reviewed and by whom
- Plan for communication or escalation if symptoms or alerts require clinical action
Common CPT 98976 Billing Mistakes (and How to Avoid Them)
❌ Billing 98976 Without a Qualifying Device
The supplied device must meet the FDA definition of a medical device and be capable of capturing and transmitting respiratory system data. Standalone apps or symptom diaries without a compliant device generally do not qualify.
❌ Fewer Than 16 Days of Data Logged
If the patient uses the device for fewer than 16 days within the 30-day monitoring window, the code may not be reported. Devices must be active and collecting data on at least 16 unique days for billing to be valid.
❌ No Documentation of Device Assignment
CPT 98976 requires documentation showing that the device was provided to the patient and that it was activated for respiratory monitoring. A general reference to “RTM enrollment” isn’t sufficient — the device and usage period must be clearly outlined.
❌ Billing Multiple Times for the Same Monitoring Period
This code is limited to once per 30-day period per patient per type of monitoring. Attempting to bill more than once for overlapping services using the same device (e.g., a pulse oximeter and spirometer combined) is not compliant unless the devices serve distinct RTM categories (e.g., musculoskeletal + respiratory).
❌ Omitting the Ordering Provider or RTM Plan
Even if the care team supplies and sets up the device, the order must come from a physician or QHP. Documentation should link the device use to a specific treatment plan and respiratory-related clinical goals.



